Enhanced solid-phase recombinase polymerase amplification and electrochemical detection
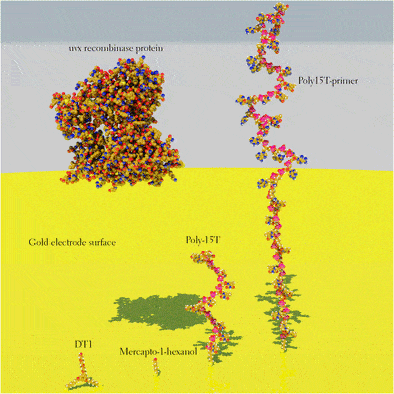
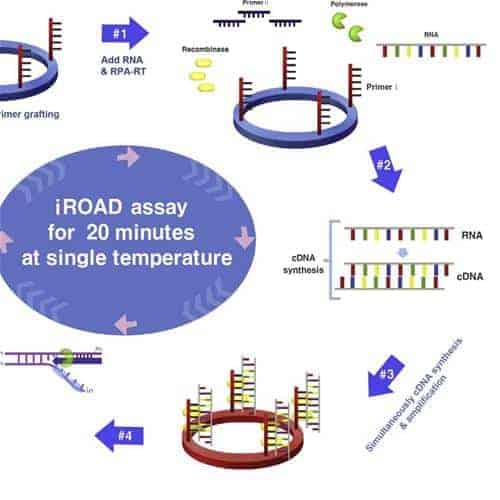
Here, we elucidate the optimal surface chemistry for rapid and efficient solid-phase RPA, which was fine-tuned in order to obtain a maximum signal-to-noise ratio, defining the optimal DNA probe density, probe-to-lateral spacer ratio (1:0, 1:1, 1:10 and 1:100) and length of a vertical spacer of the probe as well as investigating the effect of different types of lateral spacers.
Enhanced solid-phase recombinase polymerase amplification and electrochemical detection Read More